Rare earth ferroalloy
Iron alloys mainly composed of rare earth elements and iron. Additives used in steelmaking. Used in the cast iron industry as a spheroidizing agent, creep agent, and inoculant. Rare earth ferrosilicon is the main variety of rare earth ferroalloys. Rare earth ferrosilicon is not only used directly, but also as a raw material for the production of other rare earth composite alloys. The rare earth ferrosilicon (Fe SiRE) produced in China contains 20% to 47% RE, Si35% to 46%, Mn<4.0%, Ca<5.0%, Ti<3.5%, and impurities such as S, P, and C. The composition of rare earth mixed metals in Baotou, China is: Ce 44% to 46%, La16% to 18%, Pr6% to 7%, Nd27% to 29%, Sm2% to 3%, and small amounts of heavy rare earth elements such as Y, Dy, Eu, etc. There are many varieties of rare earth composite ferroalloys. Such as rare earth magnesium ferrosilicon REMgSiFe (spheroidizing agent, inoculant), rare earth calcium magnesium ferrosilicon REC; AMgSiFe (desulfurizer, purifier, creep agent), rare earth calcium ferrosilicon RECaSiFe (inoculant, creep agent), rare earth titanium magnesium ferrosilicon RETiMgSiFe (creep agent), rare earth manganese magnesium ferrosilicon REMnMgSiFe (spheroidizing agent), rare earth copper magnesium ferrosilicon RECuMgSiFe (spheroidizing agent), rare earth zinc magnesium ferrosilicon REZnMgSiFe (creep agent), etc.
Rare earth elements have been known since the 18th century, as the properties of rare earth elements were not fully understood at that time. When the mining output of ores is small, they will feel "thin". At the same time, their oxides are quite like "soil", so they are called "rare earth". Rare earth elements are the general term for the 17 elements in Group IIIB of the periodic table. Lanthanum La, cerium Ce, praseodymium Pr, neodymium Nd, giant Pm, samarium Sm, europium Eu are usually referred to as light rare earths or cerium group rare earths; Gadolinium Gd, terbium Tb, dysprosium Dy, holmium Ho, erbium Er, thulium Tm, ytterbium Yb, lutetium Lu, and yttrium Y are referred to as heavy rare earths or gadolinium group rare earths. The discovery of all rare earth elements lasted for over 150 years, from the discovery of yttrium in 1794 to the separation of uranium fission products in 1947. Since the 1950s, there have been significant breakthroughs in rare earth separation technology, with the emergence of ion exchange and solvent extraction to separate single rare earths, greatly reducing the price of rare earths and promoting research on the properties and applications of rare earth elements and their compounds. After the 1960s, the research, production, and application of rare earths entered a period of vigorous development. People have conducted extensive research on the chemical, electromagnetic, optical, and solid-state physics properties of rare earth elements, as well as metallurgical extraction processes, rare earth analysis methods, and rare earth gold phases. The discovery of their unique and excellent properties has rapidly expanded the application of rare earths to sectors such as black metallurgy, non-ferrous metallurgy, petrochemicals, glass ceramics, color television, magnetic materials, electronics industry, atomic energy industry, electric light sources and pharmaceuticals, agriculture, etc.
China began using blast furnace slag (containing RExOy 5% to 7%) from Baotou Iron and Steel Company (Baosteel) as raw material in reflector furnaces and electric arc furnaces in 1958, and using ferrosilicon as a reducing agent to produce rare earth ferrosilicon. This is the beginning of the production of rare earth ferroalloys in China. Due to the low rare earth content in blast furnace slag and the high cost of rare earth ferrosilicon, research began in 1965 by Baosteel Metallurgical Research Institute on the enrichment of rare earth slag containing RExOy 10% to 15% in a blast furnace using medium poor iron ore (containing RExOy 7% to 10%) from Bayan Obo as raw material. In 1967, the 80m3 blast furnace of Dongfeng Iron and Steel Plant in Baotou City produced rare earth slag from Baiyun Ebo's medium poor iron ore for the production of rare earth ferrosilicon and rare earth magnesium ferrosilicon. In the future, various smelting processes for rare earth composite alloys were successfully developed.
Rare earth elements are typical metallic elements. Its metal reactivity is second only to alkali metals and alkaline earth metals, and it is highly prone to form stable compounds with oxygen, hydrogen, nitrogen, sulfur, phosphorus, carbon, and other substances. The main physical properties of rare earth elements are shown in the table.
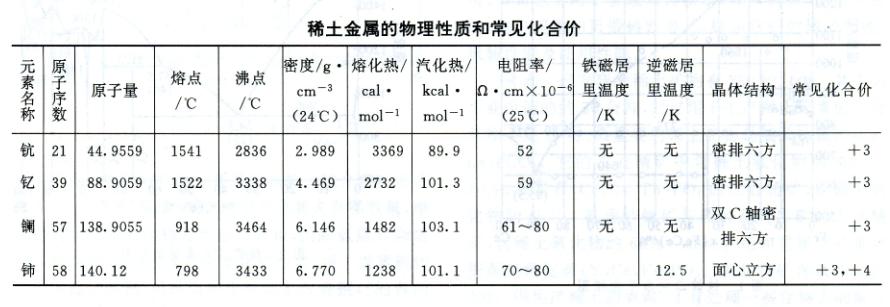
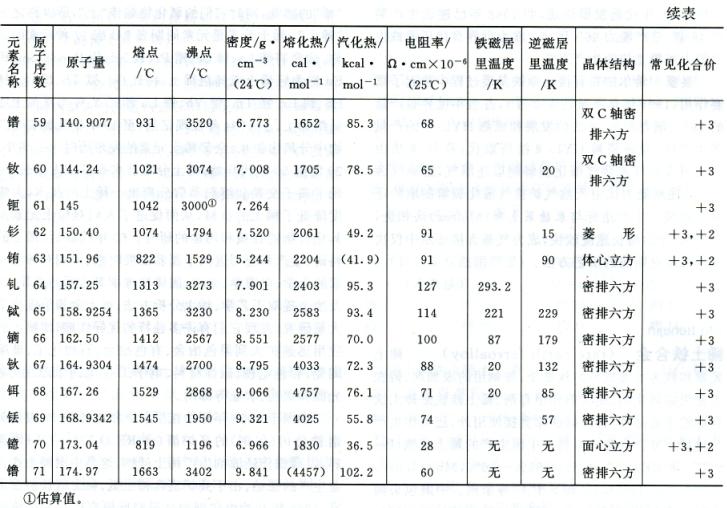
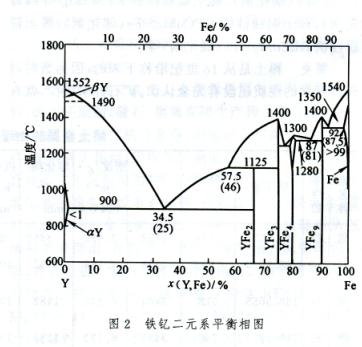
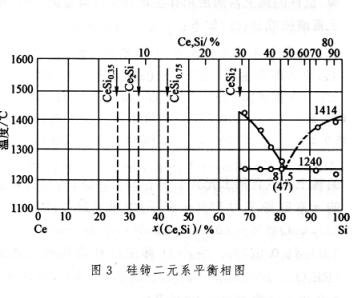
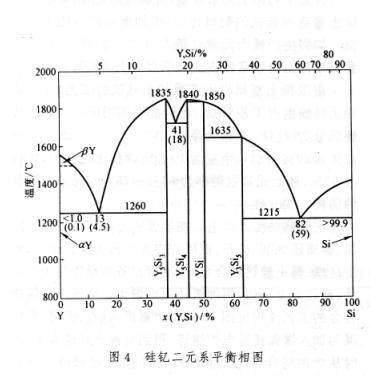
The phase diagram of binary systems composed of rare earth elements and iron or silicon can be represented by Fe Ce (Figure 1) and Fe-Y (Figure 2) or Si Ce (Figure 3) and Si-Y (Figure 4), respectively, representing the cerium group rare earths and yttrium group rare earths. Its characteristics are: (1) the solubility of rare earth elements in iron or silicon is very low; (2) Except for lanthanum, which does not form metal compounds with iron, all others form metal compounds with iron or silicon; (3) Rare earth elements dissolve with iron or silicon in liquid; (4) All have eutectic points.

The physical properties of rare earth ferroalloys produced by Chinese industry are as follows:

Rare earth metals are usually added in the form of rare earth iron alloys during steelmaking, which can remove oxygen, sulfur, reduce the number of non-metallic inclusions in steel, purify the molten steel, change the morphology and distribution of inclusions, eliminate the harm of low melting point impurities (lead, arsenic, etc.), and play a role in microalloying. Used for carbon steel and high-strength low-alloy steel, it can improve the impact toughness and anisotropy of the steel, enhance the wide cold bending qualification rate and impact performance of the plate, and reduce the tendency of cracks in the welding heat affected zone. Used for boron alloy structural steel, it can improve its low-temperature impact toughness and reduce notch sensitivity. Used for bearing steel, it can improve toughness, wear resistance, and fatigue life. Used for high-speed steel, it can improve its high-temperature plasticity. Some high-speed steels with added rare earth elements can be formed by hot twisting process. By adding appropriate amounts of rare earth elements to some ferritic and martensitic stainless and heat-resistant steels, the columnar crystal development of steel ingots can be controlled, and the quality, hot working performance, and yield of the steel can also be improved. Used for austenitic stainless and heat-resistant steel, it can improve the flowability of molten steel and the corrosion resistance of steel, improve the surface quality and thermoplasticity of steel ingots. Rare earth elements can effectively improve the high-temperature oxidation resistance and thermal strength of high chromium stainless steel; Improve the plasticity, creep resistance, and oxidation resistance of iron chromium aluminum electric heating alloys, and suppress their tendency towards high-temperature grain growth; Improve the plasticity of silicon steel used in motors and reduce its unit iron loss; Reduce the coercivity of industrial pure iron (magnetic aging can be completely eliminated when the residual rare earth content is greater than 0.05%).
Rare earth elements are extremely strong deoxidizers and desulfurizers in cast steel. It can reduce the amount of non-metallic inclusions in cast steel and change their properties and distribution. Can eliminate the harmful effects of hydrogen, nitrogen, and some low melting point impurities, thereby improving the fluidity of cast steel; Refining the as cast microstructure and eliminating porosity significantly enhance the thermal cracking resistance, plasticity, and toughness (especially low-temperature toughness) of cast steel. The use of armor cast steel can improve its casting process performance and impact toughness. Rare earth elements can alter the shape of graphite in cast iron and induce spheroidization. According to the different chemical compositions of the original molten iron and the amount of rare earth added, various shapes of graphite can be obtained, ranging from flaky, thick flaky, worm like to clustered, spherical, etc. With the change of graphite shape, the mechanical and processing properties of cast iron are significantly improved. Rare earth elements can also eliminate the adverse effects of trace elements such as titanium, antimony, bismuth, arsenic, tin, and lead in cast iron on spheroidization, and significantly reduce defects such as shrinkage porosity, slag inclusion, and subcutaneous porosity in ductile iron. Therefore, rare earth iron alloys are widely used as excellent spheroidizing and creep agents in the production of ductile iron and creep iron. 90% of ductile iron in China uses rare earth magnesium silicon iron alloy as a spheroidizing agent. In addition, rare earth elements are also commonly used in ordinary gray cast iron, high-strength gray cast iron, wear-resistant gray cast iron, white cast iron, and malleable cast iron to improve their casting performance, mechanical properties, and usability due to their harmful effects of deoxidation, desulfurization, reduction or elimination of low melting point impurities, refinement of the basic structure of cast iron, lowering the eutectic transformation temperature of cast iron, and shifting the eutectic point towards higher carbon content.
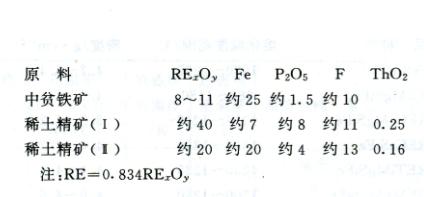
More than 250 rare earth minerals have been discovered as resources. Among them, there are about 50 types with industrial value, but less than 10 main minerals are used for the production of rare earth elements. The important rare earth mineral is fluorocarbon cerium lanthanum ore (Ce, La) FCO3. Its industrial concentrate contains about 60% to 70% rare earth oxides; The industrial concentrate of Ce, La, Th PO4 contains about 60% rare earth oxides. The industrial concentrate of fluorocarbon cerium lanthanum ore and coexisting ore contains about 60% to 68% rare earth oxides. The important resources of yttrium and heavy rare earths are yttrium phosphate (Y, Ce, Er) PO4. Industrial concentrates contain about 30% yttrium oxide. The raw materials used for the production of rare earths, as well as the rare earth concentrates produced after processing some rare earth containing composite ores, stones, and other metals such as thorium, yttrium, and single rare earth elements required for recovery.
China has extremely abundant rare earth resources, with cerium and yttrium group rare earth reserves ranking among the top in the world, and characterized by wide distribution, complete mineral types, and multiple types of mineral deposits. In addition to the Baiyun Ebo rare earth co occurring mine and the Gannan ion adsorption type mine in Inner Mongolia Autonomous Region, provinces and regions such as Guangdong, Guangxi, Jiangxi, Fujian, Hunan, Yunnan, Shandong, and Taiwan also have minerals such as pyrite, xenotime, brown yttrium niobium, silicon titanium cerium yttrium, and fluorocarbon cerium lanthanum. The rare earth raw materials for the production of rare earth ferroalloys in China are mainly sourced from the Bayan Obo mine in Inner Mongolia Autonomous Region. Their chemical composition (%) is as follows:

The enriched material obtained from chemical treatment of heavy rare earth ore used in the production of heavy rare earth ferroalloys is used as raw material. Its chemical composition is Y2O3 60%~65%, Tb4O7 3%~4%, Dy2O3 19%~20%, Ho2O3 2%~3%, other RE2O3<12%, impurities<3%.
The main method for producing rare earth ferrosilicon using the electric silicon thermal production process is shown in Figure 5. Use rare earth rich slag (RExOy>10%, ξ Fe<1.0%, Mn (1)<2%). Block size 10-100mm); Silicon iron (Si>75%, block size 10-100mm) and lime (CaO>80%, block size 10-100mm) are used as raw materials. The smelting equipment is a steelmaking electric arc furnace with feeding holes and smoke exhaust and dust removal devices on the furnace cover. Use carbonaceous furnace lining to resist the erosion of fluoride in slag. After igniting the arc, the rare earth rich slag and lime are first melted in the furnace. When the melting amount reaches 80% or more, all the ferrosilicon is added. After the furnace material is fully melted, the power is cut off; Inject compressed air (or steam) with a pressure of 0.2-0.4 MPa into the melt, stir for 3-5 minutes (in a 5-ton electric arc furnace) to accelerate the reduction reaction between silicon and rare earth oxides, and then send electricity to raise the temperature. Sample and analyze the composition of the rare earth alloy. If the rare earth content does not meet the product specifications, stir again for the second time. After passing the inspection, continue to supply power for about 10 minutes to ensure good separation of slag and iron, then cut off the power and remove the furnace. The smelting time for each furnace is about 2 hours. obtain
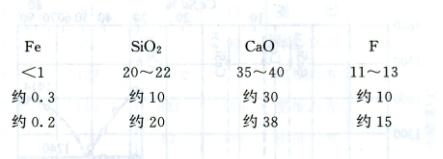
These raw materials are high in phosphorus. Rare earth rich slag with low phosphorus and low iron content should be obtained through blast furnace or electric furnace pyrometallurgical treatment and used as raw materials for the production of rare earth ferroalloys. The composition (%) of rare earth rich slag is as follows:
Rare earth ferrosilicon contains 17% to 35% rare earth elements and about 0.3% thorium. The recovery rate of rare earth elements is 55% to 65%; The composition of slag is: RExOy<2%, CaO 45%~60%, SiO2 20%~24%, F 5%~7%, ThO2 about 0.027%. The production of rare earth ferrosilicon consumes 4.0-4.5 tons of rare earth rich slag (RExOy>10%), 3.0-3.5 tons of lime, and 0.8-0.9 tons of ferrosilicon. Electricity consumption ranges from 3500 to 4000 kWh.
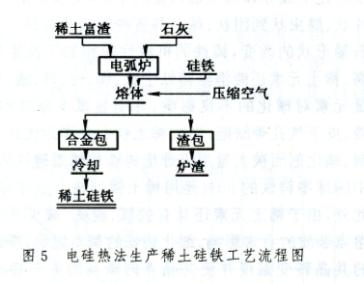
The content of rare earth elements in alloys can be adjusted by changing the ratio of rare earth rich slag to ferrosilicon and adding steel chips as needed. If it is necessary to produce rare earth ferrosilicon with a rare earth element content greater than 35%, it is necessary to use rare earth concentrate rich slag or use all rare earth concentrate rich slag.
The process of producing heavy rare earth ferrosilicon using heavy rare earth enrichment materials is similar to the production process of rare earth ferrosilicon mentioned above. The difference is that some of the 75 silicon iron needs to be replaced with calcium carbide (CaCz). The obtained alloy contains REl 8% to 45%, Si 25% to 45%, Ca2% to 8%, and the balance is Fe. Among the rare earth elements contained, Y accounts for about 55%; The yield of rare earth elements is 65% to 75%. Each kilogram of alloy consumes approximately 0.5kg of silicon.
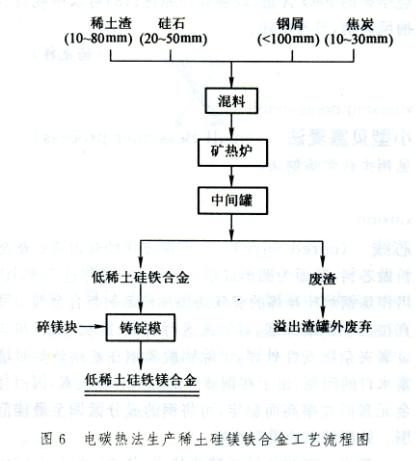
The electric carbon thermal production process directly produces rare earth ferroalloys using rare earth raw materials, carbonaceous reducing agents, silica, and steel shavings in a submerged arc reduction electric furnace. Such as rare earth ferrosilicon, rare earth silicon calcium iron alloy, etc. Smelting equipment and operations are similar to the production of ferrosilicon and silicon calcium alloys. The process flow of producing low rare earth silicon magnesium iron alloy using submerged arc reduction electric furnace is shown in Figure 6. Mix rare earth rich slag, silica, steel chips, and coke evenly and melt them in a submerged arc reduction electric furnace. Obtain low rare earth ferrosilicon alloy. Regularly place the alloy and slag into the intermediate ladle from the furnace, pour out the slag from the ladle mouth after calming, and remove the small amount of slag floating up. Place the alloy into the ingot mold containing magnesium fragments from the bottom of the intermediate package. Stir appropriately to make the ingredients uniform. The produced rare earth silicon magnesium iron alloy contains 6% to 10% RE, Mg 7%~11%, Si 44%~50%, Fe 22%~30%, Mn about 2%, Ca about 3%, Ti about 1%, Al about 0.15%, P about 0.05%. The rare earth recovery rate is about 60%.
In the early 1970s, the American company Volt Mines produced rare earth ferrosilicon alloys containing REl2% -15%, Si38% -40%, and Fe45% -52% using cerium oxide pellets, silica, steel shavings, wood shavings, and bituminous coal in 8000-8500 kW submerged arc reduction furnaces. Metallurgical workers in the former Soviet Union also conducted research on the electric carbon thermal method for smelting rare earth composite alloys.
The production process of compounding method mainly uses rare earth ferrosilicon alloy or mixed rare earth metals, single rare earth metals as raw materials, and other metal elements such as magnesium, calcium, chromium, copper, zinc, titanium, nickel are added as needed. They are remelted and mixed in a medium frequency induction furnace or crucible furnace, which is a method for producing multi-element composite rare earth ferroalloys. The alloy produced by this method has relatively pure quality, accurate composition, high element recovery rate, uniform composition, and can produce alloys with more composite elements, with high production flexibility.

